Keywords
Differentiation, Embryonic stem cell, Muscle cell, Neuron, ROCK inhibitor
Abstract
Rho kinase (ROCK) is one of the major downstream mediators of Rho. Rho plays crucial regulatory roles in the cellular proliferation and differentiation. Because a ROCK inhibitor, Y-27632 Dihydrochloride, is known to inhibit the dissociation-induced cell death in human embryonic stem (ES) cells, we investigated the effects of this ROCK inhibitor on the differentiation of the mouse ES cells. The ROCK inhibitor promoted the differentiation of the ES cells into neurons, particularly motor and sensory neurons. The addition of both ROCK inhibitor and nerve growth factor (NGF) strongly stimulated the differentiation of the ES cells into neurons. Moreover, the ROCK inhibitor promoted the differentiation of the ES cells into muscle cells. The ES cells primarily differentiated into neurons rather than muscle cells. We found that the ROCK inhibitor may promote the neuronal differentiation of the ES cells by activating the extracellular signal-regulated kinase (ERK) signaling pathway. These results suggest that the ROCK inhibitor has a significant potential to regulate the differentiation of the ES cells.
Introduction
Mouse embryonic stem (ES) cells are pluripotent cells derived from the inner cell mass of 3.5-day-old blastocysts of preimplantation mouse embryos and have a pluripotent ability to differentiate in vitro into various cell lineages, including neurons. Research efforts have been focused on ways of controlling the differentiation of the ES cells into neurons to understand the potential applications of the ES cells in neuroscience and regenerative medicine.
Watanabe et al. reported that incubation with a selective Rho kinase (ROCK) inhibitor, Y-27632, permits the survival of the human ES cells in clonal culture by inhibiting the dissociation-induced cell death. Therefore, we presumed that a ROCK inhibitor may promote the ES cell differentiation into neurons by inhibiting apoptosis. In addition, ROCKs play key roles in mediating the control of the actin cytoskeleton through the Rho family of GTPases in response to extracellular signals. Such signaling pathways contribute to diverse neuronal functions. Because ROCK regulates the function of several target proteins through its kinase activity, the inhibition of ROCK activity may provide new possibilities of controlling the in vitro differentiation of the ES cells into neurons.
In this study, to achieve the efficient differentiation of the ES cells into neurons, we investigated the effects of the ROCK inhibitor, Y-27632, and nerve growth factor (NGF) on the differentiation of the ES cells.
Materials and Methods
The Differentiation of the Mouse ES Cells
We used the mouse ES cells (129SV; Dainippon Pharmaceutical, Osaka, Japan) after 16 to 20 passages. The colony formation of the mouse ES cells was performed as described previously. To evaluate the effects of the ROCK inhibitor Y-27632 (253-00513; Wako Pure Chemical Industries, Osaka, Japan) on the ES cell differentiation, we detached the undifferentiated colonies approximately 200 micrometers in diameter from the nonadhesive 100-millimeter plastic dishes (AU2010; Eikenkizai, Tokyo, Japan). One colony per well was plated with the DMEM/F-12K medium in a gelatin-coated 96-well assay plate (353948; Becton Dickinson, Franklin Lakes, NJ, USA). The DMEM/F-12K medium consisted of 49 percent DMEM (SLM-220-B; Millipore, Temecula, CA, USA) and 49 percent F-12 nutrient mixture (21127-022; Gibco BRL, Grand Island, NY, USA), which contained 1 percent N-2 supplement (17502-048; Gibco BRL) instead of serum and 1 percent penicillin/streptomycin (15140-122; Gibco BRL). After the ROCK inhibitor or the ROCK inhibitor along with NGF (10 ng/ml; 2256X; Techne, Minneapolis, MN, USA) was added to the culture medium, the colonies were cultured for 12 days at 37 degrees Celsius in the humidified atmosphere of 5 percent CO2. Half of the medium was replaced with a fresh batch containing the ROCK inhibitor or combination of the ROCK inhibitor and NGF every 3 days.
Immunofluorescence Analysis
The mouse ES cell colonies were cultivated in a gelatin-coated 96-well assay plate, washed thrice with cold phosphate-buffered saline (PBS), and fixed with 4 percent paraformaldehyde phosphate buffer solution for 45 minutes at room temperature. After washing thrice, the cells were incubated with cold 99.8 percent methanol for 15 minutes at minus 80 degrees Celsius. The cells were incubated with primary antibodies at 4 degrees Celsius overnight. The following primary antibodies were used for labeling: anti-beta III-tubulin (MAB1637; Millipore), anti-Lim-3 (AB3202; Millipore), anti-Brn-3 (SC6026; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-alpha-actinin (sc-7453; Santa Cruz Biotechnology) antibodies. After washing thrice with cold PBS, we incubated the cells for 30 minutes at room temperature using an Alexa Fluor 488-labeled secondary antibody (A11055 or A11008; Molecular Probes, Eugene, OR, USA). After washing thrice with cold PBS, we measured the fluorescence intensity of the cells using a fluoroimage analyzer (FLA-3000R; Fujifilm, Tokyo, Japan).
Flow Cytometry
The differentiated ES cell colonies were incubated with a trypsin/EDTA solution (SM-2003-C; Millipore) for 2 minutes at room temperature and then we added DMEM to the solution. After centrifugation, the supernatant was discarded and the colonies were washed with cold PBS. The cells were dispersed by pipetting, and approximately 106 cells were transferred to a 1.5-milliliter tube. After a wash in cold PBS, the cells were incubated with 4 percent paraformaldehyde phosphate buffer solution for 30 minutes at room temperature. After centrifugation, the supernatant was discarded and the cells were washed with cold PBS. The cells were incubated with cold 99.8 percent methanol for 15 minutes at minus 80 degrees Celsius. After a wash in cold PBS, the cells were incubated with primary antibodies overnight at 4 degrees Celsius. After three washes with cold PBS, the cells were incubated with the secondary antibody for 30 minutes at room temperature. After three washes with cold PBS, we measured the number of fluorescence-activated cells using a flow cytometer (JSAN; Bay Bioscience, Kobe, Japan).
Western Blotting of Extracellular Signal-Regulated Kinase (ERK)
The ES cell colonies (10 colonies per dish) were cultured for 30 minutes at 37 degrees Celsius in the humidified atmosphere of 5 percent CO2 in a 35-millimeter culture dish (153066; Nalge Nunc International, Roskilde, Denmark) with 2 milliliters of the DMEM/F-12K medium containing 20 micromolar ROCK inhibitor and 10 ng/ml NGF. The colonies were collected and centrifuged for 5 minutes at 1500 rpm. The cell pellets were washed with 500 microliters of cold PBS. The cell pellets were lysed in 50 microliters of Pathscan sandwich ELIZA lysis buffer (7018; Cell Signaling Technology, Danvers, MA, USA). The electrophoresis was conducted under reducing conditions by standard procedures.
For western blotting, the proteins were transferred to a PVDF membrane using an electrophoretic transfer apparatus (AE6675; Atto, Tokyo, Japan). After washing, the membrane was incubated with ERK primary antibodies [an anti-p44/42 MAP kinase antibody (4695; Cell Signaling Technology) or an anti-phospho-p44/42 MAP kinase antibody (9010; Cell Signaling Technology)] overnight at 4 degrees Celsius. An ECL anti-rabbit IgG, horseradish peroxidase-conjugated, species-specific whole antibody (NA934; GE Healthcare, Buckinghamshire, UK) was used as the secondary antibody. ERK and phospho-ERK (p-ERK) were detected using an ECL Plus western blotting detection system (RPN2132; GE Healthcare). ERK and p-ERK were visualized using an ECL minicamera (RPN2069; GE Healthcare) and scanned by a GS-710 Calibrated Imaging Densitometer (Bio-Rad, Hercules, CA, USA) for quantification of protein signals on the immunoblot images.
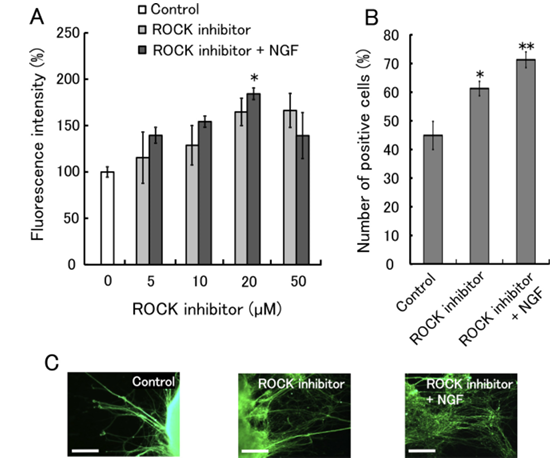
Figure 1 shows the effects of the ROCK inhibitor on the differentiation of ES cells into neurons. (A) Immunofluorescence analysis using an antibody against βIII-tubulin (a marker of postmitotic neurons) was performed. The fluorescence intensity of differentiated ES cells without ROCK inhibitor treatment was set to 100%. Data are shown as means of six replicates ± SEM. (B) The number of fluorescent cells was measured using a flow cytometer. The culture medium contained 20 µM ROCK inhibitor and 10 ng/ml NGF. Data are presented as means of three experiments ± SEM. *P < 0.05 and **P < 0.01 compared to control. (C) Fluorescence micrographs show ES cell colonies with neurite outgrowth. The cells were treated with 20 µM ROCK inhibitor and 10 ng/ml NGF, and labeled with an antibody against βIII-tubulin. Scale bar: 100 µm.
Statistical Analysis
Data were calculated as mean plus or minus SEM (standard error of the mean). Statistical comparisons were made using one-way ANOVA and the Tukey-Kramer multiple-comparison post hoc test. Values of P less than 0.05 were considered statistically significant.
Results
Effects of the ROCK Inhibitor on the Differentiation of the ES Cells into Neurons
We tested whether the ROCK inhibitor promoted the differentiation of the ES cells into neurons. Figure 1A reveals the results. The differentiated ES cells were labeled with an antibody against beta III-tubulin (a marker of postmitotic neurons). The ES cells effectively differentiated into neurons as a result of the addition of the ROCK inhibitor. Furthermore, the addition of the ROCK inhibitor and NGF strongly stimulated the differentiation of the ES cells into neurons, compared with control. Figure 1B presents the number of neurons differentiated from the ES cells. The percentage of neurons differentiated as a result of the addition of 20 micromolar ROCK inhibitor alone or both 20 micromolar ROCK inhibitor and 10 ng/ml NGF were 61 percent or 71 percent, respectively. Figure 1C presents the fluorescence micrographs of neurons differentiated from the ES cells. The ES cell colonies clearly exhibited neurite outgrowth after incubation with the ROCK inhibitor. These results are in line with results of the immunofluorescence analysis.
We characterized neurons that differentiated from the ES cells by immunofluorescence analysis. Figure 2 presents the different types of neurons. We used the antibodies against Lim-3 (a marker of cranial motor neurons) and Brn-3 (a marker of sensory neurons) because the ES cells predominantly differentiated into motor and sensory neurons. Figure 2A presents that the ROCK inhibitor promoted the differentiation of the ES cells into motor and sensory neurons. However, the effect of NGF addition on the differentiation of the ES cells into motor and sensory neurons was undetectable. Figure 2B presents the number of motor and sensory neurons. The percentage of motor or sensory neurons as a result of the addition of ROCK inhibitor were 28 percent or 24 percent, respectively. The number of the motor neurons was greater than that of the sensory neurons. Figure 2C presents the fluorescence micrographs of motor and sensory neurons differentiated from the ES cells.
Effects of the ROCK Inhibitor on the Differentiation of the ES Cells into Muscle Cells
We tested whether the ROCK inhibitor promoted the differentiation of the ES cells into muscle cells. Figure 3A presents the effects of the ROCK inhibitor on the differentiation of the ES cells into muscle cells. The differentiated ES cells were labeled with an antibody against alpha-actinin (a marker of muscle cells). The ES cells effectively differentiated into muscle cells after incubation with the ROCK inhibitor. However, the addition of the ROCK inhibitor and NGF slightly inhibited the differentiation of the ES cells into muscle cells, compared with the ROCK inhibitor alone. Figure 3B presents the number of muscle cells differentiated from ES cells. The percentage of muscle cells differentiated from ES cells after the addition of 20 micromolar ROCK inhibitor alone or both 20 micromolar ROCK inhibitor and 10 ng/ml NGF were 26 percent or 18 percent, respectively. Figure 3C presents the fluorescence micrographs of muscle cells differentiated from the ES cells. The fluorescence intensities and numbers of muscle cells agreed well. These data indicate that the ROCK inhibitor promoted the differentiation of the ES cells not only into neurons but also into muscle cells. Nonetheless, the population of cells differentiated from the ES cells consisted predominantly of neurons.
These results indicate that the ROCK inhibitor promoted the differentiation of the ES cells into neurons and muscle cells.
Detection of Phosphorylated ERK Resulting by the Addition of the ROCK Inhibitor
We investigated whether the addition of the ROCK inhibitor promoted phosphorylation of ERK in the ES cells. The ratio of p-ERK (p-ERK1 plus p-ERK2) to total ERK (ERK1 plus ERK2) was determined using western blotting as presented in Figure 4. When the ROCK inhibitor was added to the culture medium at a concentration of 20 micromolar, the ratio of p-ERK to total ERK was higher than that without the ROCK inhibitor. Furthermore, the addition of both 20 micromolar ROCK inhibitor and 10 ng/ml of NGF also promoted the phosphorylation of ERK. Furthermore, when the ES cells were treated with 1 micromolar U0126 (MAPK/ERK kinase (MEK) inhibitor; 662005; Calbiochem, Darmstadt, Germany), the 20 micromolar ROCK inhibitor did not promote the neuronal differentiation of the ES cells. MEK is an upstream ERK kinase in the ERK signaling pathway. Thus, the ROCK inhibitor may promote the neuronal differentiation of the ES cells by activating the ERK signaling pathway. However, it remains ambiguous whether the differentiation of the ES cells into muscle cells was involved in the ERK signaling pathway.
Discussion
ROCK is one of the major downstream effectors of the small GTPase Rho. Rho GTPase is involved in the regulation of neuronal morphogenesis, including migration, polarity, and axonal growth and guidance. ROCK inhibitors have been revealed to have therapeutic effects on central nervous system disorders. ROCK inhibitors could be useful at several stages of the production and use of stem cells in basic research and eventually in cell-based therapies. In this study, we evaluated the effects of ROCK inhibitor, Y-27632, and NGF on the differentiation of the ES cells, with the overarching goal being achievement of the efficient differentiation of the ES cells into neurons.
The ROCK inhibitor promoted the differentiation of the ES cells into neurons. The ES cells effectively differentiated into neurons after the addition of 20 to 50 micromolar ROCK inhibitor. Furthermore, the addition of both 20 micromolar ROCK inhibitor and 10 ng/ml NGF stimulated the differentiation of the ES cells into neurons (Figure 1). Because the number of viable cells in the case of the addition of the ROCK inhibitor alone did not increase, the ROCK inhibitor stimulated the differentiation of the ES cells into neurons. Several researchers reported the effects of the ROCK inhibitor on the neuronal differentiation of bone marrow-derived mesenchymal stem cells, mouse ES cells, adipose tissue-derived stem cells, and mouse neural stem cells. Minase et al. reported that the ROCK inhibitor potentiated NGF-induced neurite outgrowth in PC12 cells. In addition, we confirmed the promotion of neurite outgrowth in dorsal root ganglion (DRG) neurons and PC12h cells by the ROCK inhibitor. The ROCK inhibitor promoted not only the neuronal differentiation of the ES cells but also neurite outgrowth of DRG neurons and PC12h cells. The ROCK inhibitor promoted the differentiation of the ES cells into motor and sensory neurons (Figure 2). However, the addition of both NGF and ROCK inhibitor did not promote the differentiation of ES cells into motor and sensory neurons, compared with the ROCK inhibitor alone. The number of neurons other than motor and sensory neurons may increase.
These results reveal that the ROCK inhibitor stimulated the differentiation of the ES cells into neurons.
Furthermore, we found that the ROCK inhibitor also promoted the differentiation of the ES cells into muscle cells (Figure 3). The percentage of muscle cells differentiated from the ES cells after incubation with the ROCK inhibitor was 26 percent. Krawetz et al. reported that inhibition of ROCK activity promoted the differentiation of P19 cell into mesodermal and ectodermal lineages. P19 cells differentiate using the same mechanisms as normal embryonic stem cells. Because the percentage of neurons differentiated from ES cells as a result of incubation with the ROCK inhibitor was 61 percent, we concluded that the ES cells primarily differentiated into neurons.
Mitogen-activated protein kinase (MAPK) signaling pathways include two closely related pathways: MAP kinase 1 (ERK1) and 2 (ERK2). These kinases are involved in signal transduction after NGF stimulation. Activation of the MAPK signaling pathway is involved in cell survival, differentiation, and growth during neural development. Furthermore, Li et al. reported that ERK1/2 phosphorylation is a key event necessary for the early neuronal differentiation and survival of the ES cells. Therefore, we demonstrated that the addition of the ROCK inhibitor promoted phosphorylation of ERK in the ES cells (Figure 4). Furthermore, when the ES cells were treated with MEK inhibitor, the ROCK inhibitor did not promote the neuronal differentiation of the ES cells. We found that the ROCK inhibitor may promote the neuronal differentiation of the ES cells by activating the ERK signaling pathway. The addition of the ROCK inhibitor and NGF strongly stimulated the differentiation of the ES cells into neurons, compared with control (Figure 1B). Because NGF is an endogenous soluble protein that regulates survival, growth, morphological plasticity, and protein synthesis of neurons for their differentiated functions through the ERK signaling pathway, synergistic effects of NGF and ROCK inhibitor may promote the neuronal differentiation of the ES cells. However, the addition of the ROCK inhibitor and NGF slightly inhibited the differentiation of the ES cells into muscle cells, compared with the ROCK inhibitor alone (Figure 3). NGF may suppress the differentiation of the ES cells into muscle cells. Nevertheless, further studies are needed to conclude whether the ERK signaling pathway is involved in the differentiation of the ES cells into muscle cells.
Conclusions
The ROCK inhibitor promoted the differentiation of the ES cells into neurons and muscle cells. The ES cells primarily differentiated into neurons rather than muscle cells. We found that the ROCK inhibitor may promote the neuronal differentiation of the ES cells by activating the ERK signaling pathway. These results reveal that the ROCK inhibitor Y-27632 exerts a strong influence on the differentiation of the ES cells. However, significant challenges remain to be overcome for generating 100 percent pure and stable neurons.